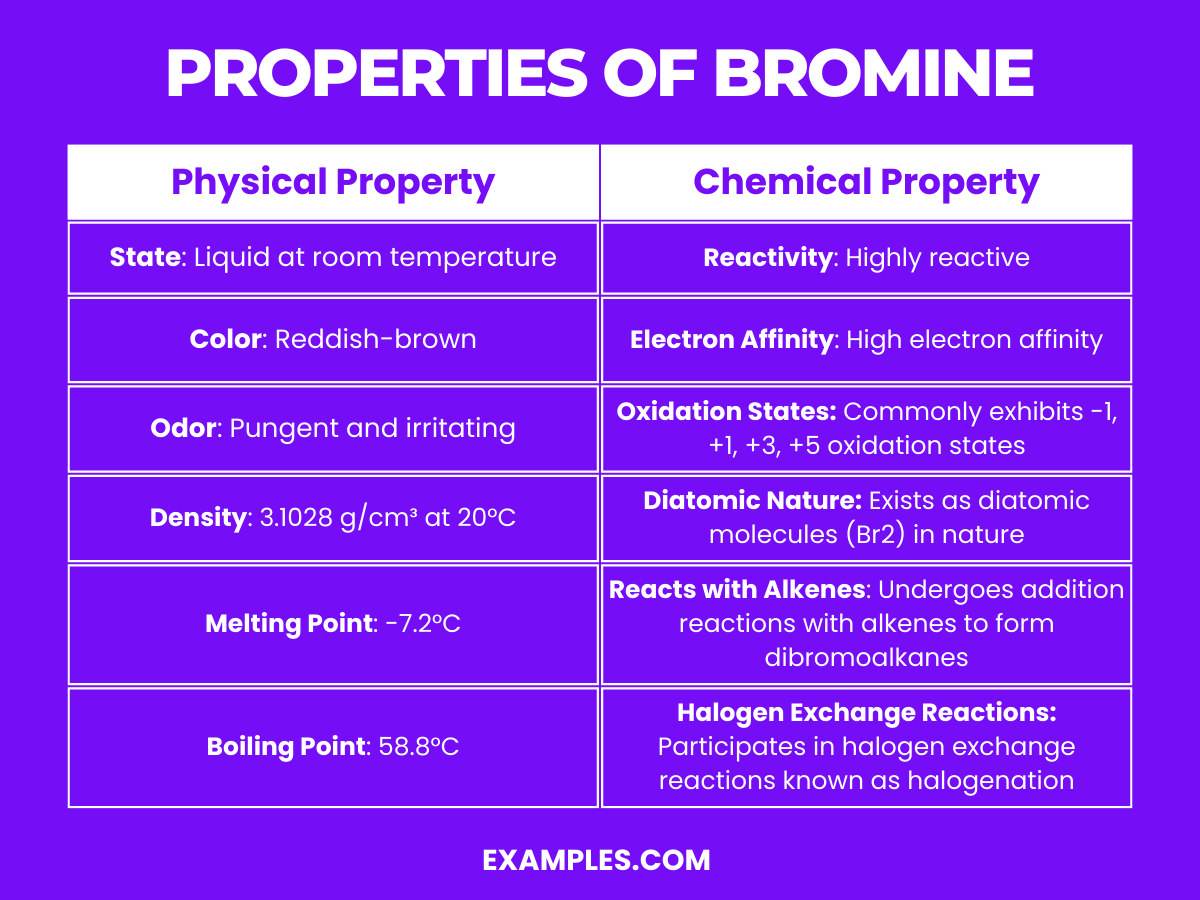
Diatomic Bromine Uses . Flushing with products with air then isolates the diatomic bromine. Diatomic bromine is a chemical compound when two neutral bromine atoms. Pure bromine is diatomic, br 2. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Like the other halogens, bromine exists as diatomic molecules in all aggregation states. Diatomic bromine, or simply bromine (br 2 ), is a molecule that forms when two bromine atoms bond. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Initiation of the reaction of bromine and toluene begins with photolytic cleavage of bromine, to give bromine radicals (br 2 + hν → 2br•).
from www.examples.com
Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Like the other halogens, bromine exists as diatomic molecules in all aggregation states. Initiation of the reaction of bromine and toluene begins with photolytic cleavage of bromine, to give bromine radicals (br 2 + hν → 2br•). Pure bromine is diatomic, br 2. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Flushing with products with air then isolates the diatomic bromine. Diatomic bromine, or simply bromine (br 2 ), is a molecule that forms when two bromine atoms bond. Diatomic bromine is a chemical compound when two neutral bromine atoms.
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds
Diatomic Bromine Uses Diatomic bromine is a chemical compound when two neutral bromine atoms. Like the other halogens, bromine exists as diatomic molecules in all aggregation states. Pure bromine is diatomic, br 2. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Diatomic bromine is a chemical compound when two neutral bromine atoms. Initiation of the reaction of bromine and toluene begins with photolytic cleavage of bromine, to give bromine radicals (br 2 + hν → 2br•). About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Diatomic bromine, or simply bromine (br 2 ), is a molecule that forms when two bromine atoms bond. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Flushing with products with air then isolates the diatomic bromine.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Diatomic Bromine Uses Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Diatomic bromine is a chemical compound when two neutral bromine atoms. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Pure bromine is diatomic, br 2. Flushing with products with air then isolates the diatomic bromine. Diatomic. Diatomic Bromine Uses.
From www.alamy.com
Chemist atom of Bromine diagram Stock Vector Image & Art Alamy Diatomic Bromine Uses Diatomic bromine, or simply bromine (br 2 ), is a molecule that forms when two bromine atoms bond. Diatomic bromine is a chemical compound when two neutral bromine atoms. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Initiation of the reaction of bromine and toluene begins with photolytic cleavage of bromine, to give bromine radicals (br. Diatomic Bromine Uses.
From www.shutterstock.com
Diatomic Bromine Molecular Structure Formula Periodic Stock Vector Diatomic Bromine Uses Like the other halogens, bromine exists as diatomic molecules in all aggregation states. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Flushing with products with air then isolates the diatomic bromine. Initiation of the reaction of bromine and toluene begins with photolytic cleavage of bromine, to give bromine radicals (br 2 + hν → 2br•). Bromine is the only nonmetallic. Diatomic Bromine Uses.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Diatomic Bromine Uses Pure bromine is diatomic, br 2. Flushing with products with air then isolates the diatomic bromine. Initiation of the reaction of bromine and toluene begins with photolytic cleavage of bromine, to give bromine radicals (br 2 + hν → 2br•). About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,.. Diatomic Bromine Uses.
From www.livescience.com
Facts About Bromine Live Science Diatomic Bromine Uses Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Diatomic bromine is a chemical compound when two neutral bromine atoms. Flushing with products with air then isolates the diatomic bromine. Like the other halogens, bromine exists as diatomic molecules in all aggregation states. Initiation of the reaction of bromine and toluene begins with photolytic cleavage of bromine, to give bromine radicals. Diatomic Bromine Uses.
From mungfali.com
Aufbau Diagram For Bromine Diatomic Bromine Uses About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Like the other halogens, bromine exists as diatomic molecules in all aggregation states. Diatomic bromine is a chemical compound when two neutral bromine atoms. Initiation of the reaction of bromine and toluene begins with photolytic cleavage of bromine, to give bromine radicals (br 2 + hν → 2br•).. Diatomic Bromine Uses.
From www.numerade.com
SOLVEDBromine is a diatomic molecule, and it has two isotopes, ^79 Br Diatomic Bromine Uses About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Flushing with products with air then isolates the diatomic bromine. Initiation of the reaction of bromine and toluene begins with photolytic cleavage of bromine, to give bromine radicals (br 2 + hν → 2br•). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Diatomic bromine, or simply bromine (br. Diatomic Bromine Uses.
From www.sciencephoto.com
Bromine, atomic structure Stock Image C018/3716 Science Photo Library Diatomic Bromine Uses About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Like the other halogens, bromine exists as diatomic molecules in all aggregation states. Diatomic bromine, or simply bromine (br 2 ), is a molecule that forms when two bromine atoms bond. Diatomic bromine is a chemical compound when two neutral bromine atoms. Initiation of the reaction of bromine. Diatomic Bromine Uses.
From brainly.com
the shape and bonding in a diatomic bromine molecule is best described Diatomic Bromine Uses About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Pure bromine is diatomic, br 2. Flushing with products with air then isolates the diatomic bromine. Diatomic bromine, or simply bromine (br 2 ), is a molecule that forms when two bromine atoms bond. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Initiation of the reaction of bromine. Diatomic Bromine Uses.
From www.slideserve.com
PPT Bromine PowerPoint Presentation, free download ID2813065 Diatomic Bromine Uses Initiation of the reaction of bromine and toluene begins with photolytic cleavage of bromine, to give bromine radicals (br 2 + hν → 2br•). Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Flushing with products with air then isolates the diatomic bromine. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Diatomic bromine, or simply bromine (br. Diatomic Bromine Uses.
From techiescientist.com
11 Uses of Bromine That You Must Know Techiescientist Diatomic Bromine Uses Initiation of the reaction of bromine and toluene begins with photolytic cleavage of bromine, to give bromine radicals (br 2 + hν → 2br•). Like the other halogens, bromine exists as diatomic molecules in all aggregation states. Diatomic bromine, or simply bromine (br 2 ), is a molecule that forms when two bromine atoms bond. Flushing with products with air. Diatomic Bromine Uses.
From www.thoughtco.com
What Are the 7 Diatomic Elements? Diatomic Bromine Uses About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Diatomic bromine, or simply bromine (br 2 ), is a molecule that forms when two bromine atoms bond. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Flushing with products with air then isolates the diatomic bromine. Diatomic bromine is a chemical compound when two neutral bromine atoms. Like. Diatomic Bromine Uses.
From www.shutterstock.com
Diatomic Bromine Properties Chemical Compound Structure Stock Vector Diatomic Bromine Uses Diatomic bromine, or simply bromine (br 2 ), is a molecule that forms when two bromine atoms bond. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Pure bromine is diatomic, br 2. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Like the other halogens, bromine exists as diatomic molecules in all aggregation states. Diatomic bromine is. Diatomic Bromine Uses.
From www.britannica.com
bromine Properties, Uses, & Facts Britannica Diatomic Bromine Uses Pure bromine is diatomic, br 2. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Diatomic bromine is a chemical compound when two neutral bromine atoms. Diatomic bromine, or simply bromine (br 2 ), is a molecule that forms when two bromine atoms bond. Flushing with products with air. Diatomic Bromine Uses.
From www.indiamart.com
Liquid Diatomic Bromine, for Laboratory, Rs 160/liter Sri Sai Diatomic Bromine Uses Pure bromine is diatomic, br 2. Diatomic bromine, or simply bromine (br 2 ), is a molecule that forms when two bromine atoms bond. Like the other halogens, bromine exists as diatomic molecules in all aggregation states. Diatomic bromine is a chemical compound when two neutral bromine atoms. Bromine is the only nonmetallic element that is liquid at ordinary temperatures.. Diatomic Bromine Uses.
From www.youtube.com
Intermolecular Forces for Br2 (Diatomic Bromine) YouTube Diatomic Bromine Uses Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Like the other halogens, bromine exists as diatomic molecules in all aggregation states. About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Flushing with products with air then isolates the diatomic bromine. Diatomic bromine, or simply bromine (br 2 ), is a molecule that forms when two bromine atoms. Diatomic Bromine Uses.
From sciencenotes.org
What Are the 7 Diatomic Elements? Definition and List Diatomic Bromine Uses Pure bromine is diatomic, br 2. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Like the other halogens, bromine exists as diatomic molecules in all aggregation states. Initiation of the reaction of bromine and toluene begins with photolytic cleavage of bromine, to give bromine radicals (br 2 + hν → 2br•). Diatomic bromine, or simply bromine. Diatomic Bromine Uses.
From www.examples.com
Bromine (Br) Definition, Preparation, Properties, Uses, Compounds Diatomic Bromine Uses About 3.41 grams (0.12 ounce) of bromine dissolve in 100 milliliters (0.1. Pure bromine is diatomic, br 2. Flushing with products with air then isolates the diatomic bromine. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Diatomic bromine is a chemical compound when two neutral bromine atoms. Like the other halogens, bromine exists as diatomic molecules in all aggregation states.. Diatomic Bromine Uses.